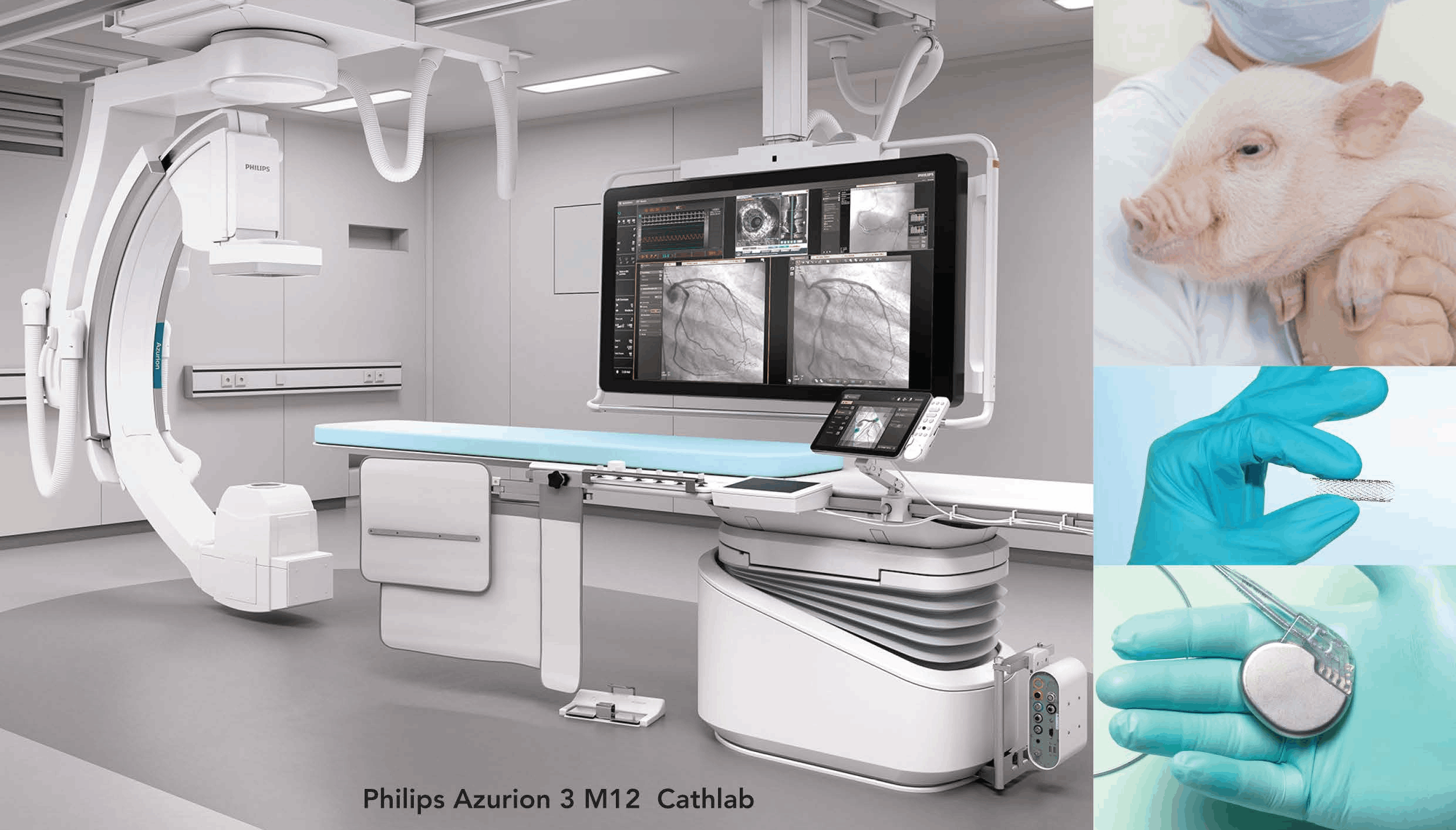
Preclinical services for performance, biocompatibility & safety evaluation of given medical devices and therapeutics (ISO 10993, ISO 25539-2: 2012 and 21 CFR 58 / GLP)
Cardiovascular segment devices
- Cardiac rhythm / Flow management devices (Defibrillator & Pacemaker)
- Interventional cardiac devices (Stents, Catheters & Angioplasty balloons)
- Cardiac monitoring / diagnostic devices (Telemetry systems)
- Peripheral vascular devices (Stents, Grafts & Balloon catheters)
- Electrophysiological devices (RF ablation & laser catheters, 3D Mapping systems)
- Prosthetic devices and ventricular function devices (Prosthetic valves & VAD)
Gastrointestinal segment devices
- Nasogastric Tubes
Genitourinary segment devices
- Urinary incontinence devices (macroplastique, urethral sling implants & nephrostomy tubes / urinary stents )
Neurological segment devices
- Neuroplasty implants (Cochlear implants)
- Renal denervation ablation devices (RDN catheters)
Dental devices
- Endosteal / Endosseous
- Single-Stage and subperiosteal dental implants
- Subperiosteal dental implants
Integumentary implants devices /
therapeutics
therapeutics
- Hemostats & Sealants
- Cellular implant therapies for wound healing
Orthopedic segment
- Tissue engineered grafts for bone defects (Arthroplasty tissue implants)
- Knee replacement implants (Metal and Ceramic implants)