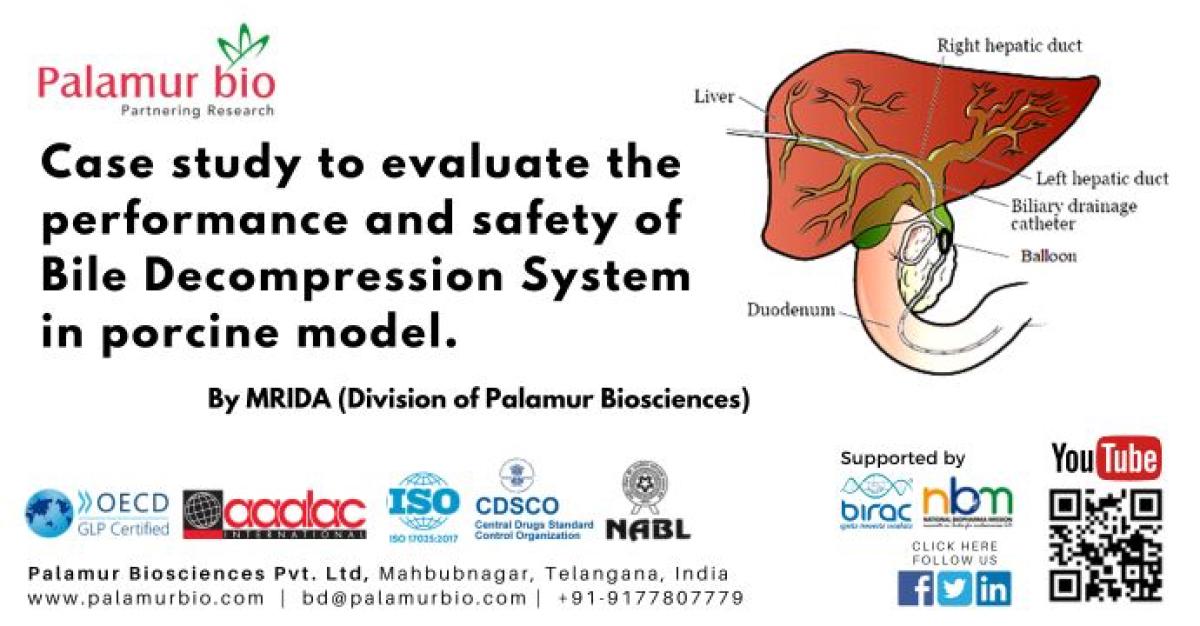
Bile is a fluid made by your liver. It helps break down food. The bile flows from liver through bile ducts to small intestine. When the bile duct is narrowed or blocked by scar tissue or a tumor, bile can no longer flow into the first part of your small intestine, called the duodenum (see Figure given below). This causes the bile to collect in the liver. The build-up of bile in liver can cause nausea, vomiting, fever, itching, and particularly jaundice.
If the bile duct is blocked, a biliary drainage catheter is placed to allow the bile to drain from the liver and connected to a motor pump or drainage bag.
The current study involves use of the Bile Removal Pump assembly - 4Fr-6Fr Introducer Sheath, Balloon dilation catheter to create the biliary obstruction followed by biliary drainage.
Testing to be done on 6 Pigs. (Yorkshire, pink, ~4 months old, 3 female &3 male)
Test Method: In-vivo testing in a porcine model will be conducted to validate the ability of the newly developed system to provide biliary decompression in a porcine biliary obstruction model.
Expected Outcomes: Fluoroscopic imaging to document biliary dilation and decompression, - blood tests confirming decreasing serological markers for biliary obstruction - documentation of adverse event rates, including catheter occlusion and entry site leakage.
Minimum acceptance criteria and the Metric(s) for success: Demonstrating the ability to repeatedly create an obstructed biliary model, the ability to demonstrate both the provision and maintenance of biliary decompression on fluoroscopic imaging and decreased levels of serum total bilirubin, alkaline phosphatase and gamma glutamyl transferase as compared to pre-device implantation.
The present study will utilize a porcine model for biliary obstruction created by using an internal external biliary catheter with an inflatable balloon. An internal-external biliary drainage catheter together with the balloon goes through the skin and into your bile ducts to create a blockage the balloon is kept inflated. One end of the catheter will sit in your small intestine, and the other will come out of your body and will be attached to a drainage bag or motor pump. This catheter lets bile flow in 2 directions, either out to the external collecting bag or into your small intestine. This is the most common kind of drainage catheter (See the figure given below)
On Day #1 Endoscopically the opacification of the intrahepatic biliary system will be done. With the use of the cathlab percutaneous biliary puncture under fluoroscopy, following guidewire and catheter exchanges a sheath will be inserted.
The modified percutaneous transhepatic internal external catheter will be inserted coaxially via this sheath. Once the catheter is inserted contrast will be used to position the catheter so that it straddles the Ampulla of Vater. The balloon, situated within the midportion of the perforations of the catheter, will be inflated within the common bile duct. Contrast injected via the side arm of the sheath will be used to confirm that the balloon inflation is occlusive to flow via the common bile duct into the duodenum. A cholangiogram will be performed with or without the coaxial insertion of the Bile Saver device in its activated state and the behavior of the contrast in the biliary system will be assessed. After 1 Hr of system operation, fluoroscopic imaging will be repeated to confirm biliary decompression. –
Blood work will be used to track serum levels on days 1, 3, 5 during the experiment. On the 3rd day, contrast enhanced fluoroscopy will be used to repeat the study performed on Day #1. After 1 hrs of system operation, fluoroscopic imaging will be repeated to confirm biliary decompression.
The study will run for a total of 30 days with additional fluoroscopic images taken on day 30. While primary end-point for the study are improved serum Total Bilurubin, ALK ,GGT levels combined with fluoroscopic imagery to assess and confirm biliary decompression. Secondary endpoints will include daily visual inspection of catheter connections, catheter entry site leakage, and confirmation of pump operation.
Scheduled Euthanasia: All the Animals will be humanely euthanized on day 30 to collect limited tissues for the local safety of the hepatobiliary system together with the heart, lung and kidneys.
Histopathology: The balloon inflated region of the together with the whole biliary ducts and liver and the adjoining muscles will be harvested for the local safety evaluation through histopathology. Other limited organs will be lung liver kidneys and heart will also be collected for histopathology to evaluate related microscopic lesions.
For More details please contact us at bd@palamurbio.com