Ocular implants play a crucial role in treating various eye conditions in animals. The ocular fundus, a significant part of the eye, includes the upper tapetal fundus (if present), the ventral and surrounding nontapetal fundus, retinal vasculature, and optic disc. The posterior segment of the ocular fundus comprises structures such as the posterior sclera, choroid (containing pigmented cells, blood vessels supporting the outer retina's high metabolic needs, and the tapetum lucidum to enhance vision in dim light), retina (with nine layers of neurosensory retina and the outer retinal pigment epithelium), and the optic disc. The optic disc is where retinal ganglion axons exit the eye through a weak and fenestrated scleral lamina cribrosa to synapse in the lateral geniculate body for vision or the midbrain and rostral colliculi for reflex responses (such as pupillary light reflex or dazzle reflex).
The anatomy of the eye differs across various animal species. For example, animals like dogs, cats, horses, cattle, sheep, goats, and many others possess an upper tapetal fundus, while pigs and rabbits usually do not have a tapetal fundus. Moreover, minipigs (pigs in general) have puncta only on the upper eyelid, and rabbits have them exclusively on the lower eyelid. These distinctions are crucial to take into account when considering the implantation of ocular implants in animals.
Rabbits are commonly used as an animal model for ocular research due to their relatively large eyes and ease of handling. They have gained favour for their numerous advantages, including:
-
Docility: Rabbits are non-aggressive and easy to handle and observe.
-
Economy: Rabbits are widely bred and very economical compared with the expense of larger animals.
-
Short vital cycles: Rabbits have short vital cycles (gestation, lactation, and puberty).
Pigs and Sheep are both used as large animal models for ocular research due to their similarities with human eyes in terms of size and anatomy. Some of the advantages and disadvantages of using pigs and sheep as animal models for ocular research are:
Advantages of using pigs as an animal model for ocular research:
-
Anatomical similarities: The ocular anatomy of pigs is similar to that of humans, making them a good model for studying human eye diseases.
-
Ease of handling: Pigs are relatively easy to handle and can be trained to cooperate with researchers.
-
Availability: Pigs are widely bred and readily available, making them a cost-effective animal model for ocular research.
The only physiological disadvantage of Pig model is that pigs have a higher intraocular pressure than humans, which can affect the results of experiments involving ocular implants .
Advantages of using sheep as an animal model for ocular research:
-
Anatomical similarities: The ocular anatomy of sheep is similar to that of humans, making them a good model for studying human eye diseases .
-
Large size: Sheep are larger than many other animal models used in ocular research, which makes them useful for certain types of experiments .
-
Availability: Sheep are widely bred and readily available, making them a cost-effective animal model for ocular research .
Sheep is not having any anatomical or physiological disadvantage. This makes the sheep as gold standard model for the evaluation of Ocular implants.
The regulatory requirements for preclinical studies for Ocular Implants vary depending on the country and the type of implant.
- The International Organization for Standardization (ISO) has published guidelines for ocular implants.
-
ISO 16672:2020 specifies requirements for Ocular Endotamponades, a group of non-solid surgically invasive medical devices introduced into the vitreous cavity of the eye to flatten and position a detached retina onto the retinal pigment epithelium (RPE), or to tamponade the retina. The document specifies requirements for their intended performance, design attributes, pre-clinical and clinical evaluation, along with the other information supplied by the manufacturer.
-
ISO 11979-5:2020 specifies requirements for the preclinical requirements of Intraocular Lenses.
-
- ANSI® Z80.27-2014 (R2019) is an American National Standard for Ophthalmics which provide the information on the testing requirements for Implantable Glaucoma Devices.
MRIDA is the Medical Devices Testing and Training Facility of Palamur Biosciences, a well acclaimed Pre-Clinical CRO which complies with various national and International Quality systems such as OECD-GLP, ISO17025, AAALAC, etc. MRIDA (www.PBSMRIDA.com), mainly focused on Cardiothoracic/ Vascular/ other Surgical Interventional Medical Devices testing offering invivo efficacy and safety studies with most modern infrastructure and Equipments.
This Blog is the first Case Study of the Ocular Implant describing the procedural details of the safety evaluation of an Implantable Glaucoma Drainage Device.
Animal: Rabbit / New Zealand White / Male
Animal Preparation: The animal was anaesthetized by injecting Ketamine (30mg/Kg) and Xylazine (5mg/Kg) intramuscularly in thigh muscle and kept for maintenance anaesthesia with inhalational Isoflurane (1 %).
The animal was then placed on the operating table and draped. Procedures were carried out under an operating microscope using aseptic techniques.
Implantation Procedure: Unilateral implantation was carried out in all the animals. In right eye Implantable Glaucoma Drainage Device was implanted while sham surgery was carried out in contralateral left eye.
Below is the stepwise procedure for the implantation of Implantable Glaucoma Drainage Device:
1. On Right eye conjunctival peritomy, incision was made and a pocket was formed superiorly, avoiding the superior rectus and oblique muscles, with blunt dissection of Tenon’s capsule from the episcleral.
Fig 2: Incision at the site of implantation
2. The drainage plate was placed under the conjunctiva and tenon’s capsule between the rectus muscles and sutured to the episclera. The leading edge of the endplate was ~ 10 mm from the limbus.
Fig 3: Drainage plate Implantation
3. A sharp needle was inserted into the anterior chamber at 1-2 mm way from the limbus at an angle parallel to the iris plane. The nozzle for the Test Drainage implant was inserted all the way into the anterior chamber through the ostium created by the needle. The Test Drainage implant was secured in place within the sclera by stitching in the rear of the device through the two stitch holes using a suitable suture with a round-shaped needle.
Fig 4: Implantation of Test Drainage Implant
4. The tube of the drainage plate was then connected to the rear end connector of the Test Drainage implant (called outlet).
5. A scleral patch was then placed and sutured in place.
Fig 5: Suturing at the implanted site with Scleral patch.
6. Finally, the conjunctiva was carefully closed using an appropriate suturing technique.
Fig 6: Closing of conjunctiv.
Control eyes (contralateral): Sham surgery was carried out on the control eye (left eye) that is as similar to implantation surgery as possible without implantation of the device.
The sham surgery was performed as described below:
-
A conjunctival peritomy incision was made and a pocket was formed superiorly, avoiding the superior rectus and oblique muscles, with blunt dissection of Tenon’s capsule from the episcleral.
-
A sharp needle was inserted into the anterior chamber at 1-2 mm way from the limbus at an angle parallel to the iris plane.
-
Two stitches was performed within the sclera using a suitable suture with a round-shaped needle.
-
Finally, the conjunctiva was carefully closed using an appropriate suturing technique.
As a post-operative care, a dosing regimen of analgesics and antibiotics was started immediate after surgery till 10 days.
Both eyes of each animal were observed using Slit-lamp microscope and Indirect ophthalmoscope on Day 0 (before surgery), 1, 3, 7, 14, 28, 14th week and 26th week. After completion of 26 weeks, the eyes were enucleated, and the implants were retrieved. The histopathology evaluation was carried out for the enucleated eyes.
The Histopathology Images as given below:
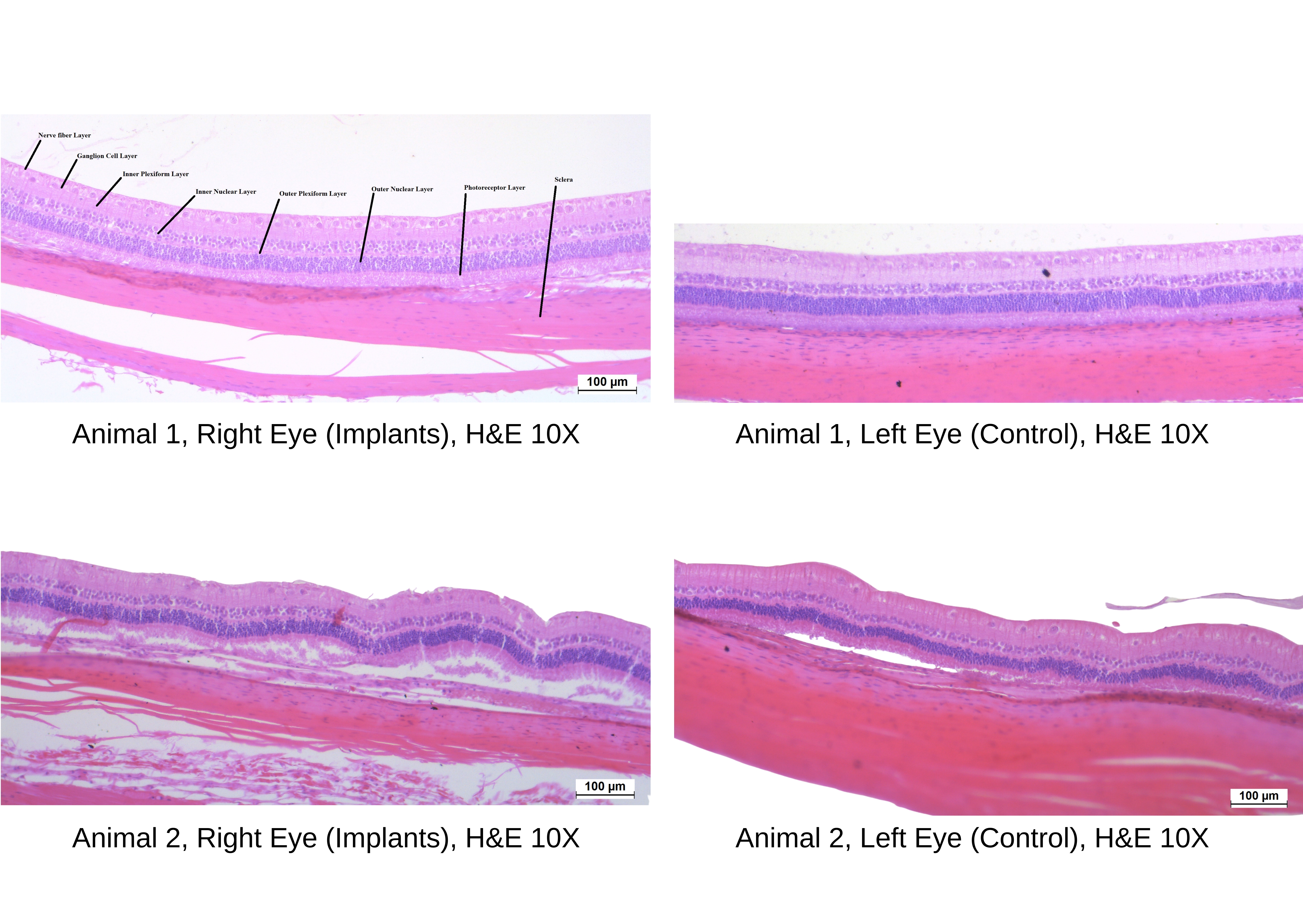
RESULTS: All the animals showed normal intraocular pressure when compared to the control eye till 26-week post-implantation.
Slit-lamp biomicroscopy did not revealed any abnormality in the pupillary light reflexes, conjunctiva, cornea, aqueous and vitreous humor, iris and lens till 26-week post-implantation. The evaluation of fundus using indirect ophthalmoscope showed normal blood vessels, optic disc and macula with normal fovial reflex till 26-week post-implantation.
In the gross necropsy observations, no abnormality detected in the right eye with the implants as compared to concurrent control left eye.
In the retrieved implants, encapsulation and vascularization was observed in the right eye with the implants in all the animals which is common finding for this type of implants. The microscopic examination of control and implant eye were within normal limit.
Stay in tune for more case studies of other ocular implantable devices……
For More details please contact bd@palamurbio.com / Click Here to Whatsapp



